What Are The Biotic And Abiotic Factors That Limit Population Size?
Abstract
Biotic and abiotic factors are increasingly acknowledged to synergistically shape wide-scale species distributions. However, the relative importance of biotic and abiotic factors in predicting species distributions is unclear. In particular, biotic factors, such as predation and vegetation, including those resulting from anthropogenic land-use change, are underrepresented in species distribution modeling, but could improve model predictions. Using generalized linear models and model choice techniques, nosotros used 129 estimates of population density of wild pigs (Sus scrofa) from 5 continents to evaluate the relative importance, magnitude, and management of biotic and abiotic factors in predicting population density of an invasive large mammal with a global distribution. Incorporating diverse biotic factors, including agriculture, vegetation comprehend, and large carnivore richness, into species distribution modeling substantially improved model fit and predictions. Abiotic factors, including precipitation and potential evapotranspiration, were besides of import predictors. The predictive map of population density revealed wide-ranging potential for an invasive large mammal to aggrandize its distribution globally. This information tin can exist used to proactively create conservation/management plans to control future invasions. Our study demonstrates that the ongoing paradigm shift, which recognizes that both biotic and abiotic factors shape species distributions across wide scales, can be advanced past incorporating diverse biotic factors.
Introduction
Predicting and mapping species distributions, including geographic range and variability in abundance, is fundamental to the conservation and management of biodiversity and landscapes1. The ecological niche defines species-habitat relationships2,3,iv and provides a useful framework for understanding the range and abundance of species in relation to biotic and abiotic factors. Farther, niche relationships beyond local scales can provide novel information about the ecology, conservation, and management of species at macro scalesfive. Most studies evaluating a species' niche across their distribution focus on presence-absence occurrence information to predict the geographic rangehalf dozen; however, conservation and management plans for species can exist improved past agreement patterns of population abundance and density across a species' range7. In particular, evaluating population density, compared to occurrence, can reveal novel patterns of species distributions in relation to landscape factors8.
There is an ongoing paradigm shift in understanding how biotic and abiotic factors shape species distributions. Until recently, it was widely accepted that abiotic factors, such as temperature and precipitation, played the master role in shaping distributions of species and biodiversity at wide scales (e.chiliad., regional, continental, global extents) and that biotic factors were most important at fine scales (e.g., site, local extents)9,ten,11. Information technology is increasingly recognized, however, that biotic factors are important determinants of species distributions at broad spatial scales, especially when considering biotic interactions12,xiii,14,15,xvi. Although interspecific competition can be an important biotic determinant in species distribution models at broad scales, other forms of biotic interactions, such as predation and symbioses, tin can likewise be important determinantsfifteen,17, but take received less attending18. In addition, although researchers take evaluated the effects of biotic interactions on geographic range limitsxviii, relatively few studies have evaluated how biotic factors influence population density across a species' range19,20, which can be more informative in understanding macro-ecological patterns7,21.
In improver to species interactions, biotic factors related to vegetation can influence species distributions and abundance at wide scales. In particular, anthropogenic land-use modify is rarely considered when evaluating species distributions at broad scales; still, given the human footprint globally22 and projections for expanding human impacts on the surroundings23,24, biotic factors created by human activities are potentially important predictors that tin contribute to a better understanding of species distributions8. For example, agricultural crops are a ascendant biotic factor beyond continents that are facilitated by homo engineering and the redistribution of ecological resource and energy, which tin can have profound impacts on constitute and animal populations beyond broad extents; agriculture can increase populations for some species through increased food, resources availability, and landscape heterogeneity, or decrease populations due to loss of habitat25,26,27. Ultimately, further evaluation is necessary to sympathize the relative importance of abiotic and biotic factors in shaping species distributions across broad spatial scalesthirteen,15.
Invasive species are a primary driver of widespread and severe negative impacts to ecosystems, agronomics, and humans across local to global scales28. These introduced plants and animals often exhibit broad geographic distributions, can be relatively well studied across local scales, and provide novel opportunities to evaluate broad-scale patterns of niche relationships29. Predictions of potential geographic distribution of invasive species tin provide critical information that can inform the prevention, eradication, and command of populations, which has been evaluated for many taxa, including plants30, amphibians31, and invertebrates32. However, few studies have predicted the potential ranges and abundance of non-native mammals33. Especially for broad-ranging species that tin occur beyond broad extents of landscapes, predictions of how population density varies spatially can provide important information for prioritizing conservation and management deportment.
Few species showroom a global distribution that extends across Europe, Asia, Africa, North and South America, Australia, and oceanic Islands. Besides naturalized animals, such as the house mouse (Mus musculus) and brown rat (Rattus norvegicus), wild pigs (Sus scrofa; other common names include wild boar, wild/feral swine, wild/feral grunter, and feral grunter) take ane of the widest geographic distributions of whatever mammal; farther, information technology exhibits the widest geographic range of any large mammal34, with the exception of humans. The expansive global distribution of wild pigs is attributed to its broad native range in Eurasia and northern Africa, widespread introduction by humans exterior its native range, and superior adaptability, where information technology occurs in a wide diverseness of ecological communities, ranging from deserts to temperate and tropical environments35,36, with a corresponding diverse omnivorous nutrition37. Across its non-native range (Fig. 1; Supplementary Methods S1), including Northward and South America, Australia, sub-Saharan Africa, and many islands, wild pigs are considered one of the 100 most harmful invasive species in the earth38 due to wide-ranging ecosystem disturbance, agronomical harm, pathogen and disease vectors to wild animals, livestock and people, and social impacts to people and property39,40,41. Wild pigs are therefore a model species to evaluate biotic and abiotic factors associated with population density because they exhibit a global distribution across six continents, are widely studied across much of their native and not-native (i.e., invasive or introduced) ranges, and previous research has indicated that their population density was related to abiotic factors across a continental scale, although it was ambiguous how biotic factors shape their abundance, warranting further written report42.

Areas of white betoken locations in which wild pigs are likely not present. This map was created using ArcGIS x.3.i98. Meet Supplementary Methods S1 for a description of methods and citations used for creating the map of wild sus scrofa global distribution beyond its native and not-native ranges.
To address these ecological questions and understand the relative importance of biotic and abiotic factors in shaping the global distribution of a highly invasive mammal, we evaluated estimates of population density of wild pigs across various environments on five continents. Specifically, we (1) evaluate how biotic (i.e., vegetation and predation) and abiotic (i.e., climate) factors (Table ane) shape population density across a global scale and (2) create a predictive distribution map of potential population density across the world. We besides compare population density betwixt island and mainland populations. Our results contribute novel insight into the relative roles of biotic and abiotic factors in shaping the distribution of species' population densities across continental and global scales, particularly relating to human-mediated land-use modify, which can provide critical information to management and conservation strategies.
Results
We compiled 147 estimates of wild hog density (# animals/kmtwo), which resulted in 129 estimates of density across their global distribution used in our analyses (Fig. i; Supplementary Table S2). Some areas contained > ane density gauge, and these were averaged. Population density of wild pigs was college on islands (due north = xi) compared to on the mainland (n = 118) (t = 4.72, df = 10.93, p < 0.001; Supplementary Effigy S3). For the untransformed density estimates, mean population density for on the mainland equaled 2.75 (se = 0.38) and islands equaled 18.52 (se = four.xv). The highest estimates of population density occurred on islands, which reached upwards of 40 wild pigs/km2 (Supplementary Table S2). Due to differences in population density betwixt islands and on the mainland, we used density estimates from mainland populations in our subsequent analyses.
Population density was influenced by both biotic and abiotic factors across the global distribution (Tables two and 3; Supplementary Table S4). The suite of all-time models all included combinations of biotic and abiotic factors (Table ii) and the top model (AICc = 237.94; model weight = 0.68; adjusted R2 = 0.55) had > 1,000 times more back up every bit the all-time approximating model than the top model because only abiotic factors (AICc = 311.30; model weight = 7.94 × 10−17) (Supplementary Table S4). The variables with the greatest importance included potential evapotranspiration, big carnivore richness, precipitation during the wet and dry out seasons, unvegetated area, and agriculture, which too exhibited 95% conviction intervals that did not overlap zero (Table 3). Density was greatest at moderate levels of potential evapotranspiration and agriculture, decreased with big carnivore richness and amount of unvegetated area, and increased with precipitation during the wet and dry seasons (Fig. 2); pct forest embrace was unsupported in models when considering the suite of variables in analyses.
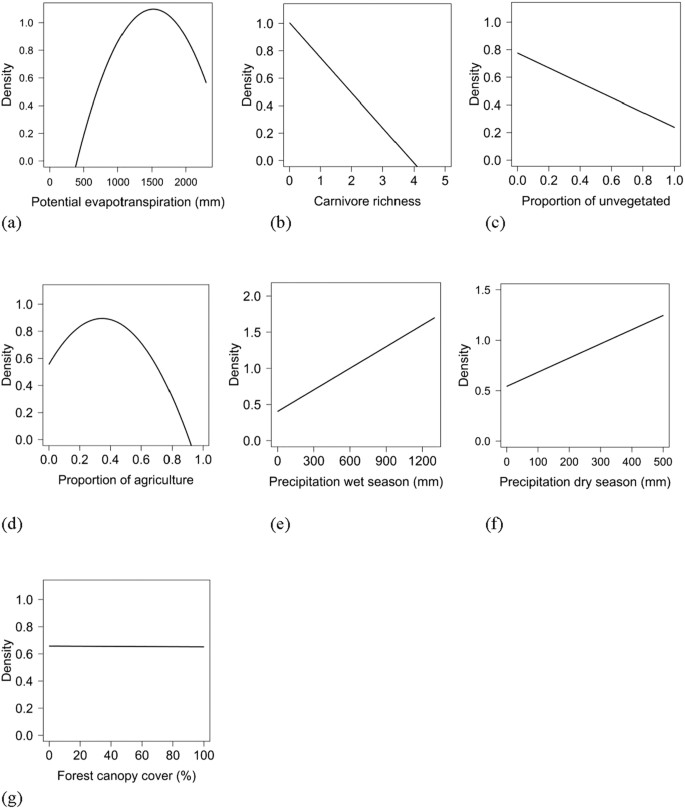
Relationships of biotic and abiotic factors with population density (natural log calibration; #/km2) of wild pigs, including potential evapotranspiration (a), large carnivore richness (b), unvegetated (c), agriculture (d), precipitation during the wettest flavor (east), precipitation during the driest season (f), and woods awning cover (g).
Using the total model-averaged results of parameter estimates, we created a predictive map of global wild pig population density (Fig. 3; Supplementary Effigy S5). Wild pig populations were predicted to occur at low to high population densities across all continents, including large areas of state where wild pigs are currently absent. The highest predicted densities occurred in southeastern, eastern, and western North America, throughout Central America, northern, eastern, and southwestern South America, western, southern, and eastern Eurasia, throughout Indonesia, central and southern Africa, and northern and southeastern Australia (Fig. 3; Supplementary Figure S5). Results of k-fold cross validation demonstrated that the model had expert predictive power with a hateful squared prediction error (MSPE) of 0.22 and a Pearson's correlation between observed and predicted values of 0.lxxx (t = 17.711, df = 181, p-value < 0.001).
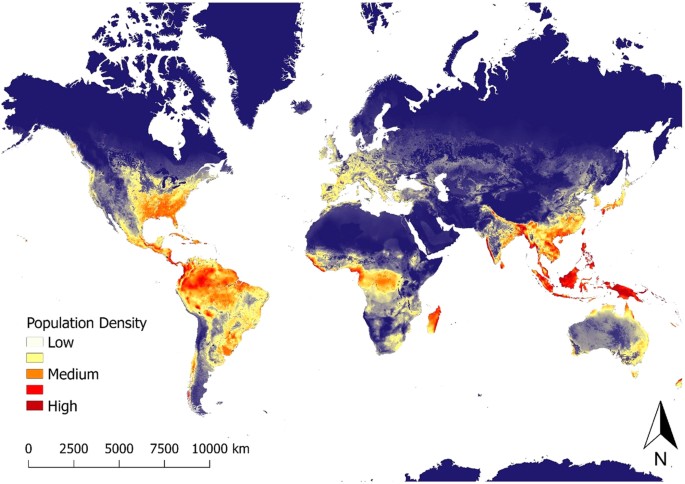
For terrestrial environments, areas of white represent low density (1 individual/km2), orange moderate density (6 individuals/km2), and night cherry high density (≥eleven individuals/kmii). Maps were created using Google Earth Engine80 and QGIS 2.xiv.iii90. See Supplementary Figure S5 for finer scale maps of predicted population density of wild pigs for Europe, Asia, Africa, Commonwealth of australia, North America, and South America.
Word
Population density of an invasive large mammal was strongly influenced by both biotic and abiotic factors across its global distribution. Consistent with the prediction that abiotic factors drive broad-scale patterns of species distribution, potential evapotranspiration (PET) and atmospheric precipitation variables were important predictors of population density on a global scale. In addition, contributing to growing evidence that biotic factors are also important determinants of broad-scale patterns of species distributions, both biotic interactions and vegetation played of import roles in predicting the distribution of wild grunter populations globally. Farther, land-use change mediated past man activities strongly predicted the broad-scale distribution of an invasive large mammal. Consistent with previous studies evaluating how population density of ungulates varied across wide scales, both bottom-up (resource-related) and height-downwards (predation) factors influenced the distribution of wild pig populations19,42,43. Ultimately, wild sus scrofa populations across their global distribution appeared to answer to biotic and abiotic factors related to plant productivity, provender and h2o availability, cover, predation, and anthropogenic land-use change.
Using both biotic and abiotic factors to evaluate broad-scale species distributions can create more realistic maps of range and density with improve predictive ability16,44, which can better inform management and conservation strategies for species. For example, population density of wild pigs was highest in landscapes with moderate levels of agriculture and PET, lower large carnivore richness and amount of unvegetated area, and greater precipitation during the wet and dry seasons. Using these relationships, we created a predictive map of population density across the globe, which can be used to manage existing populations and predict areas where wild grunter populations are likely to expand or invade if given the opportunity. Ultimately, this data can exist used to prioritize management activities in areas at risk of invasion and with expanding populations.
Abiotic factors, such every bit temperature and atmospheric precipitation, are consistently found to be primary determinants of species distributions at broad scales11. Potential evapotranspiration can be peculiarly informative for understanding wide-scale ecological patterns45, such equally species distributions. This was supported in our enquiry where PET was the most important predictor of population density beyond the global distribution of wild pigs. Potential evapotranspiration is highly correlated with temperature variables, thus indicating that wild sus scrofa density was greatest at relatively moderate temperatures and density was lower in areas exhibiting extreme low and high temperatures. In addition, the stiff support of precipitation variables in our models is consistent with the association of wild pigs with vegetation comprehend, forage, and water36. In particular, precipitation probable facilitates rooting behavior by wild pigs by softening the soil substrate46.
Biotic factors were amid the most supported variables predicting population density across a global scale. Our results indicated that the presence of large carnivores tin influence wild pig population density. Big carnivore richness was strongly supported in our models and exhibited a negative human relationship with wild squealer density; as the number of large carnivore species increased, wild sus scrofa density decreased, which is consistent with studies in Eurasia and Australia42,47,48. In add-on, interspecific competition can influence the distribution of species and it has been hypothesized that wild pigs have not extensively invaded wildlands in some regions of sub-Saharan Africa due to the presence of other pig species that showroom like niches49. Although contest with other species might influence wild pig populations and their distribution49,l,51, in other cases wild pigs are reported to spatially and temporally partition habitat use to reduce niche overlap with potential competitors52,53,54 and not show evidence for interference contest with related mammals (e.g., species within the suborder Suiformes), such as native peccary species55, thus, it is unclear how interspecific interactions influence wild pig populations beyond their global distribution. Further, agreement potential interspecific contest for invasive species can be especially challenging in non-native habitat because invaders have not coevolved with competitors or predators and thus it is difficult to predict which species will exist subordinate or dominant in potential competitive interactions or how competition might influence species distributions in unoccupied habitat17,xviii,56. Because it was unknown how competitive interactions between wild pigs and other species might influence their distribution, particularly outside their native range, competition was not included in our analyses. To understand how competition between non-native and native species influences species distributions, field studies evaluating interspecific competition are necessary beyond the wild pig's native and not-native geographic range, particularly beyond local spatial scales.
Although biotic interactions between animals are the principal biotic factors evaluated in species distribution models at broad scales, the office of constitute communities has received less consideration. In detail, anthropogenic land-use change increasingly influences vegetation communities across continents and warrants a meliorate understanding for how human activities are shaping broad-calibration distributions of plant and animal populations22,24. For example, agriculture is a dominating land cover type across continents23,25, which can potentially benefit species distributions in at to the lowest degree two ways. Agriculture tin can (ane) increment population density within areas of a species' electric current geographic range through supplemental food and increased resource availability and (2) allow geographic ranges to expand past creating habitat in areas that were previously unsuitable. In contrast, as agriculture increasingly dominates landscape patterns at broad extents, cover and other resources correspondingly decrease, which tin negatively impact the geographic range and population density of some species. Our results demonstrate that agriculture can produce both positive and negative effects on populations, depending on the levels of agriculture. At intermediate levels of agriculture, population density of wild pigs was greatest, likely due to an optimal mix of nutrient and cover. Whereas, at high levels of agriculture, population density decreased precipitously, which was probable a result of inadequate cover. Our results indicate that heterogeneous landscapes with a mix of agronomics and cover will support the greatest populations of wild pigs, which is consistent with broad-scale patterns of wild pig populations in North America and Eurasia57,58,59. Due to relatively high predicted population densities of wild pigs inhabiting heterogeneous landscapes, these regions would probable experience the greatest crop damage, leading to high economic loss to farmers.
Forest is considered a key habitat type preferred by wild pigs59,60. In univariate analyses, forest was an important positive predictor of wild pig density (β = 0.170, se = 0.056). When considering boosted predictor variables in our models, notwithstanding, forest was relatively unimportant in predicting wild pig density, which is also consistent when evaluating wild pig occurrence over broad scales57. Thus, the estimation of how wood influences the distribution of wild pigs must be considered in the context of other variables included in models, where abiotic factors might adequately explain forest distribution (run into discussion below). However, as predicted, vegetation and cover play a strong role in predicting wild pig density; as the amount of unvegetated area increased across the landscape, wild pig population density decreased, which is consistent with geographic distribution maps of wild pigs61.
In some systems, abiotic factors can be stronger predictors of species distributions, than biotic factors, because of loftier correlations between these ii factors62. Our study indicated that both factors can exist of import predictors of species distributions, potentially because abiotic factors may poorly predict biotic factors stemming from human activities. In addition, homo influences might weaken the correlation between abiotic and biotic factors. For example, humans tin can significantly reduce the number of large carnivores in an area63, although these species would exist predicted to occur across broad areas based on abiotic factors and celebrated biotic atmospheric condition. In addition, human land use change can lead to unpredictable biotic patterns in relation to abiotic factors, such every bit through agricultural landscape conversion. Although soil types might support crop production, many agricultural areas occur in arid landscapes requiring irrigation of water and application of fertilizer to maintain production25. Thus, agricultural crops could not abound in many areas based on wide-scale climate factors lone, and therefore, abiotic factors tin be poor predictors of agronomical practices in some regions. Indeed, there likely are other examples where abiotic and biotic factors may showroom low correlation in some systems (eastward.grand., location of human activities and evolution, altered interspecific interactions due to human activities, and other forms of anthropogenic country utilise change). Ultimately, it can be useful to consider biotic factors in species distribution models that might be poorly predicted by abiotic factors due to human activities.
Additional biotic factors that can influences species distributions on a broad scale, peculiarly invasive species, include the role of humans in distributing the founding individuals of new populations. For example, invasive wild pig populations have arisen beyond several continents recently through man activities. Illegal translocations by humans for hunting purposes can facilitate the long-altitude expansion of wild grunter populations into new areas64,65,66, which is currently a primary source of new populations globally39,41. Further, in countries such as Canada, Brazil, and Sweden, wild hog farms were the propagule source for contempo populations of wild pigs across broad regions, which are currently spreading into new areas67,68,69. Indeed, propagule pressure (i.due east., the number of individuals introduced and release events) determines both the likelihood of invasive species condign established, as well equally the charge per unit of geographic range expansion60,lxx. In addition, invasive species that exhibit r-selected characteristics (eastward.g., early maturity, short generation time, and high fecundity) can be more probable to successfully invade novel landscapes71. Fifty-fifty at depression population densities, invasive species with high reproductive output are more likely to constitute populations in areas of lower quality habitat72. Given that wild pigs are one of the well-nigh fecund large mammals (e.1000., hateful litter sizes ranging from 3.0 to 8.iv piglets per sow with the potential for >ane litter annually)36, their reproductive characteristics might increase the probability of establishment and enable them to compensate for small population sizes when introduced into novel environments across a range of habitat qualities.
Population density, compared to presence-absence occurrence, tin provide more informative conclusions of species distributions in relation to biotic and abiotic factors7,viii. For example, although large carnivores probable do not exclude wild pigs from habitat beyond broad scales, our study revealed they can influence abundance. However, occurrence of species would remain abiding across varying population densities, unless it resulted in species exclusion. Ultimately, population densities tin provide more detailed information about species distributions, which tin can better inform conservation and management plans and policyvii. Studies analyzing presence-only data with logistic regression and Maximum Entropy (MaxEnt) models have examined methods to accost spatial sampling bias73,74,75 and additional evaluations would exist useful for studies using population density data with multiple linear regression. Farther, global analyses of population genetics could be used to place groups and the proportion of wild and domestic genes across wild pig populations, which could be used to comprise population construction into analyses to better understand population characteristics.
Predicting species distributions provides critical information to the management and conservation of biodiversity, especially for controlling invasive species. Without intensive direction actions, our study predicts that in that location is strong potential for wild pigs to expand their geographic range and farther invade expansive areas of North America, South America, Africa, and Australia. Although wild pigs currently occupy broad regions of predicted habitat in their not-native range, many regions of predicted habitat are currently unoccupied and may be at loftier adventure for future invasion. These areas might warrant increased surveillance past local, state, and federal agencies to counter the establishment of populations. Although attending in unoccupied areas that are predicted to support high densities of wild pigs might warrant priority for countering population introductions, wild pigs can persist in relatively low quality habitat (e.g., arid and/or cold regions) and these areas also warrant attention to halt invasions. Given the potential for wild sus scrofa populations to rapidly expand one time established36, predictions of potential population density in unoccupied habitat can provide critical information to land managers, which can be used to proactively develop management plans to prevent introductions and control or eradicate populations if they become introduced.
Methods
Density Estimates
To evaluate the population density (i.e., number of individuals per unit expanse) of wild pigs throughout their global distribution, we compiled density estimates from the literature throughout its native and non-native ranges across each continent and island for which data were available (Supplementary Table S1). Previous research evaluated how population density of wild pigs varied across western Eurasia42 and nosotros incorporated these 54 estimates of population density into our analysis. In improver, we followed the methodological recommendation of Melis et al.42. to average data when multiple estimates were available for >1 season or year at a report surface area. Island populations typically exhibit higher population density compared to mainland populations76,77. We thus compared estimates of wild sus scrofa population density between isle and mainland populations; if population density for islands was significantly college than on the mainland, we focused on only evaluating mainland populations in subsequent analyses.
Models evaluating and predicting species distributions tin can be improved by including areas of absence (a.thousand.a., pseudo-absenteeism or background locations) or cypher density to sample the full range of available mural conditionsi to predict the potential range of a species, absenteeism locations should occur outside the ecology domain of the species, but inside a reasonable altitude of the species' geographic range78. Because wild pigs have occurred inside their native range for thousands of years, we assumed that populations were at equilibrium and the species had colonized available habitat associated with its geographic distribution. Thus, regions adjacent to its native distribution that were classified every bit unoccupied were assumed to be unsuitable for population persistence due to unfavorable environmental weather condition. In addition, spatial sampling bias (i.e., uneven sampling across geographic extents) can be addressed by increasing the number of groundwork locations in areas with greater sampling73,74. The majority of density estimates used in our study occurred within the wild pig's native range of Europe and Asia and we focused sampling of background locations associated with this region. To include locations with estimates of zero density in our analyses, we used a three-stride arroyo. Get-go, nosotros created a buffered region that occurred across the area between 100–m km around the purlieus of the wild pig's native range79. Side by side, we calculated the spatial extent of the native range and buffered regions. Lastly, accounting for the expanse of each region, nosotros selected a random sample of locations within the buffered region that was proportional to the number of estimates used in the native terrestrial range of wild pigs. Based on this approach, we used 65 locations of zero density in our analyses that occurred across fundamental Russian federation, Mongolia, western Red china, Kingdom of saudi arabia, and northern African countries. Nothing density estimates were used in analyses relating wild pig density to landscape variables and excluded when comparing population density betwixt island and mainland populations.
Landscape Variables
We considered a suite of biotic and abiotic mural variables, which were divided into vegetation, predation, and climate factors (Table 1) that we hypothesized to influence population density of wild pigs. We used mural variables that were bachelor globally and, where possible, over long fourth dimension periods (i.e., estimates averaged over several decades) that coincide with the density estimates nosotros compiled for our analyses. Geospatial data layers were acquired through either Google World Engine80 or were downloaded from online sources (Table ane).
The biotic factors that nosotros evaluated included agronomics, broadleaf wood, enhanced vegetation index (EVI), forest awning embrace, difference in the proportion between forest and agriculture (to characterize landscape heterogeneity), normalized divergence vegetation index (NDVI), large carnivore richness, and unvegetated area (Tabular array i). We expected a positive human relationship between density and all vegetation factors, except unvegetated area, due to their association with increased food availability, establish productivity, and cover. In addition, we expected a quadratic relationship betwixt population density and agriculture because we predicted density to be greatest at moderate levels of agriculture (due to a mix of encompass and food) and low at high levels of agronomics (due to a lack of adequate comprehend). Finally, we expected a negative relationship between population density and large carnivore richness.
The abiotic factors that we evaluated included two measures of ecological energy regimes, actual evapotranspiration (the corporeality of h2o loss from evaporation and transpiration, which is related to plant productivity) and potential evapotranspiration (PET; the amount of evaporation and transpiration that would occur with a sufficient water supply, considering solar radiation, air temperature, humidity, and wind speed;45). Actual evapotranspiration is a measure of h2o-energy residual and potential evapotranspiration is considered a measure of ambient energy and often highly correlated with temperature variables81. Although evapotranspiration variables can include elements of biotic (i.due east., transpiration from plants) and abiotic (i.e., climate and water) factors, they were classified as abiotic for our analyses. In addition, we evaluated precipitation during dry and wet seasons, and annually, and temperature during summer and wintertime, and annually (Tabular array i). Nosotros predicted a positive relationship between density and precipitation variables due to associated increases in forage, water, and encompass and quadratic relationships between density and evapotranspiration and temperature variables due to expected peak densities at intermediate levels and low densities at depression and high levels.
Modeling
We used data from the wild sus scrofa's native and non-native range in our modeling. Although niche shifts between a species' native and non-native range appear to be uncommon and it is often causeless that species exhibit niche stasis or conservatism30,82,83,84 through space and time, models that use data but from a species' native range can exhibit poor predictive power in the species' non-native range85,86,87. Therefore, information technology is of import to include data from the species' entire distribution to increment the predictive ability of models beyond both the native and non-native ranges32,88,89. Considering wild pigs take been established across much of their non-native range for an extended period of fourth dimension (e.g., typically greater than a century), nosotros causeless that populations used in our analyses had achieved a localized equilibrium with their environment.
All geospatial data layers were evaluated using QGISxc and Google Earth Engine80 and statistical analyses were conducted using R91. Because there is uncertainty nigh the exact location of studies and the calibration in which processes might influence wild pig densities, we evaluated multiple scales for each covariate using 10, 20, and twoscore km radius buffers effectually the location of each density estimate (Table 1). Thus a moving window approach was conducted so that each pixel inside a spatial layer summarized the landscape within the buffered radius. To determine the best scale for analyses we used a multi-criteria arroyo. First, variables were centered and scaled to amend model fit92. Next, nosotros considered quadratic relationships for mural factors that were predicted to exhibit a curvilinear pattern (Table 1). Concluding, nosotros selected the best scale and relationship for each covariate based on wild squealer ecology, model comparisons using Akaike's Data Criterion corrected for small sample size AICc;93, and plots of residuals. Once the appropriate calibration was determined for each variable (Table ane), we evaluated the Pearson correlation amid all variables and excluded highly correlated variables (r > 0.70) from our final assay.
We used multiple linear regression to evaluate how population density was influenced by our concluding suite of biotic and abiotic factors (Tabular array 1). The distribution of density estimates were correct skewed, thus nosotros log-transformed density estimates using the natural logarithm42. To compare the relative importance of biotic and abiotic factors and to determine parameter estimates of variables, we ranked all possible models using AICc, model-averaged parameter estimates (i.e., total provisional), and calculated variable importance values93,94,95. We used model weights and evidence ratios to evaluate if biotic factors improved model fit by comparing models including only abiotic factors to models also including biotic factors. Model averaged parameter estimates were used to create a predictive global map of wild sus scrofa density (one kmtwo resolution). This map displays the maximal potential density of wild pigs in relation to the biotic and abiotic factors used in our modeling and reflects predicted densities that would be accomplished if wild pigs had access to all landscapes, their movements were unrestricted, and management activities did not suppress populations. We validated our model using mean squared prediction fault (MSPE)96 and k-fold cantankerous validation and selected the number of bins based on Huberty's rule of pollex (k = 4)97.
Boosted Data
How to cite this commodity: Lewis, J. S. et al. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. vii, 44152; doi: 10.1038/srep44152 (2017).
Publisher's annotation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
Franklin, J. Mapping species distributions: spatial inference and prediction. (Cambridge University Printing, 2009).
-
Grinnell, J. The niche-relationships of the California Thrasher. The Auk 34, 427–433 (1917).
-
MacArthur, R. H. In Population Biology and Development(ed R. C. Lewontin ) 159–186 (Syracuse University Press, 1968).
-
Hutchinson, G. E. Concluding remarks. Common cold Spring Harbor Symposium on Quantitative Biology 22, 415–427 (1957).
-
Chocolate-brown, J. H. Macroecology: progress and prospect. Oikos 87, 3–14 (1999).
-
Elith, J. & Leathwick, J. R. Species distribution models: ecological caption and prediction across space and fourth dimension. Annual Review of Ecology, Evolution, and Systematics twoscore, 677–697 (2009).
-
Brown, J. H., Mehlman, D. Due west. & Stevens, Chiliad. C. Spatial variation in abundance. Ecology 76, 2028–2043 (1995).
-
Randin, C. F., Jaccard, H., Vittoz, P., Yoccoz, N. Grand. & Guisan, A. Land apply improves spatial predictions of mount plant abundance but not presence-absence. Journal of Vegetation Scientific discipline 20, 996–1008 (2009).
-
Pearson, R. G. & Dawson, T. P. Predicting the impacts of climatic change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12, 361–371 (2003).
-
Benton, 1000. J. The Red Queen and the Court Jester: species multifariousness and the role of biotic and abiotic factors through time. Science 323, 728–732 (2009).
-
Wiens, J. J. The niche, biogeography and species interactions. Philosophical Transactions of the Purple Society of London B: Biological Sciences 366, 2336–2350 (2011).
-
Van der Putten, Westward. H., Macel, Thousand. & Visser, M. E. Predicting species distribution and abundance responses to climatic change: why it is essential to include biotic interactions across trophic levels. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2025–2034 (2010).
-
Meier, E. South. et al. Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography 33, 1038–1048 (2010).
-
Guisan, A. & Thuiller, Westward. Predicting species distribution: offering more than unproblematic habitat models. Ecology Messages 8, 993–1009 (2005).
-
Wisz, M. S. et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews 88, 15–thirty (2013).
-
Leach, Chiliad., Montgomery, Due west. I. & Reid, N. Modelling the influence of biotic factors on species distribution patterns. Ecological Modelling 337, 96–106 (2016).
-
Anderson, R. P. When and how should biotic interactions exist considered in models of species niches and distributions? Journal of Biogeography, doi: ten.1111/jbi.12825 (2016).
-
Sexton, J. P., McIntyre, P. J., Angert, A. L. & Rice, K. J. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40, 415–436 (2009).
-
Melis, C. et al. Predation has a greater impact in less productive environments: variation in roe deer, Capreolus capreolus, population density beyond Europe. Global Environmental and Biogeography 18, 724–734 (2009).
-
Pasanen-Mortensen, M., Pyykönen, M. & Elmhagen, B. Where lynx prevail, foxes will fail–limitation of a mesopredator in Eurasia. Global Ecology and Biogeography 22, 868–877 (2013).
-
Boulangeat, I., Gravel, D. & Thuiller, W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecology Letters 15, 584–593 (2012).
-
Sanderson, E. Due west. et al. The Human being Footprint and the Terminal of the Wild. Bioscience 52, 891–904 (2002).
-
Laurance, W. F., Sayer, J. & Cassman, Thousand. K. Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Development 29, 107–116 (2014).
-
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
-
Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. (ESA Working Paper No. 12-03, Rome, FAO, 2012).
-
Green, R. E., Cornell, Due south. J., Scharlemann, J. P. & Balmford, A. Farming and the fate of wild nature. Science 307, 550–555 (2005).
-
Bengtsson, J., Ahnström, J. & Weibull, A.-C. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. Journal of Applied Ecology 42, 261–269 (2005).
-
Mack, R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10, 689–710 (2000).
-
Parmesan, C. et al. Empirical perspectives on species borders: from traditional biogeography to global alter. Oikos 108, 58–75 (2005).
-
Peterson, A. T. Predicting the geography of species' invasions via ecological niche modeling. The Quarterly Review of Biology 78, 419–433 (2003).
-
Ficetola, Yard. F., Thuiller, W. & Miaud, C. Prediction and validation of the potential global distribution of a problematic alien invasive species—the American bullfrog. Diverseness and Distributions xiii, 476–485 (2007).
-
Sánchez-Fernández, D., Lobo, J. M. & Hernández-Manrique, O. L. Species distribution models that do not incorporate global data misrepresent potential distributions: a instance study using Iberian diving beetles. Diverseness and Distributions 17, 163–171 (2011).
-
Kauhala, Thou. & Kowalczyk, R. Invasion of the raccoon domestic dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Current Zoology 57, 584–598 (2011).
-
Oliver, West. L. R. & Brisbin, I. In Pigs, peccaries and Hippos: status survey and conservation action plan(ed Westward. L. R. Oliver ) 179–195 (IUCN, 1993).
-
Oliver, West. L. R., Brisbin, I. L. & Takahashi, S. In Pigs, peccaries and Hippos: status survey and conservation activity plan(ed W. 50. R. Oliver ) 112–120 (IUCN, 1993).
-
Mayer, J. & Brisbin, I. L. Wild pigs: biology, damage, command techniques and management. (Savannah River Site Aiken, SC, USA, 2009).
-
Ballari, S. A. & Barrios-García, M. N. A review of wild boar Pig diet and factors affecting food selection in native and introduced ranges. Mammal Review 44, 124–134 (2014).
-
Lowe, S., Browne, M., Boudjelas, Due south. & De Poorter, M. i 00 of the world's worst invasive alien species: A pick from the global invasive species database. 1–12 (Aukland, New Zealand, 2000).
-
Barrios-Garcia, M. Northward. & Ballari, Due south. A. Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14, 2283–2300 (2012).
-
Courchamp, F., Chapuis, J.-L. & Pascal, Thou. Mammal invaders on islands: impact, command and control bear upon. Biological Reviews 78, 347–383 (2003).
-
Bevins, S. N., Pedersen, K., Lutman, M. W., Gidlewski, T. & Deliberto, T. J. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 64, 291–299 (2014).
-
Melis, C., Szafrańska, P. A., Jędrzejewska, B. & Bartoń, K. Biogeographical variation in the population density of wild boar (Hog) in western Eurasia. Journal of Biogeography 33, 803–811 (2006).
-
Danell, Thousand., Bergström, R., Duncan, P. & Pastor, J. Big herbivore environmental, ecosystem dynamics and conservation. Vol. eleven (Cambridge University Press, 2006).
-
González-Salazar, C., Stephens, C. R. & Marquet, P. A. Comparing the relative contributions of biotic and abiotic factors as mediators of species' distributions. Ecological Modelling 248, 57–70 (2013).
-
Fisher, J. B., Whittaker, R. J. & Malhi, Y. ET come dwelling house: potential evapotranspiration in geographical ecology. Global Ecology and Biogeography xx, ane–xviii (2011).
-
Sandom, C. J., Hughes, J. & Macdonald, D. West. Rooting for rewilding: quantifying wild boar's Grunter rooting rate in the Scottish Highlands. Restoration Ecology 21, 329–335 (2013).
-
Woodall, P. F. Distribution and population dynamics of dingoes (Canis familiaris) and feral pigs (Sus scrofa) in Queensland, 1945-1976. Journal of Applied Environmental twenty, 85–95 (1983).
-
Ickes, Thousand. Hyper-abundance of native wild pigs (Squealer) in a lowland Dipterocarp rain wood of peninsular Malaysia Biotropica 33, 682–690 (2001).
-
Oliver, Due west. & Fruzinski, B. In Biology of Suidae(eds R. H. Barrett & F. Spitz ) 93–116 (Institute National de Recherche Agronomique, Castanet, France, 1991).
-
Jedrzejewska, B., Jedrzejewski, W., Bunevich, A. N., Milkowski, L. & Krasinski, Z. A. Factors shaping population densities and increment rates of ungulates in Bialowieza Primeval Woods (Poland and Belarus) in the 19th and 20th centuries. Acta Theriologica 42, 399–451 (1997).
-
Corbett, L. Does dingo predation or buffalo competition regulate feral sus scrofa populations in the Australian moisture-dry torrid zone? An experimental written report. Wildlife Inquiry 22, 65–74 (1995).
-
Ilse, 50. M. & Hellgren, East. C. Resource partitioning in sympatric populations of collared peccaries and feral hogs in southern Texas. Journal of Mammalogy 76, 784–799 (1995).
-
Desbiez, A. L. J., Santos, S. A., Keuroghlian, A. & Bodmer, R. East. Niche partitioning among white-lipped peccaries (Tayassu pecari), collared peccaries (Pecari tajacu), and feral pigs (Squealer). Journal of Mammalogy xc, 119–128 (2009).
-
Gabor, T. M., Hellgren, Eastward. C. & Silvy, Due north. J. Multi-calibration habitat partition in sympatric suiforms. The Periodical of Wildlife Management 65, 99–110 (2001).
-
Oliveira-Santos, L. Thousand., Dorazio, R. M., Tomas, W. M., Mourao, 1000. & Fernandez, F. A. No evidence of interference competition amid the invasive feral hog and two native peccary species in a Neotropical wetland. Journal of Tropical Environmental 27, 557–561 (2011).
-
Louthan, A. M., Doak, D. F. & Angert, A. Fifty. Where and when do species interactions set range limits? Trends in Ecology & Evolution xxx, 780–792 (2015).
-
McClure, M. L. et al. Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS I ten, e0133771 (2015).
-
Massei, G. et al. Wild boar populations upwards, numbers of hunters down? A review of trends and implications for Europe. Pest Management Science 71, 492–500 (2015).
-
Honda, T. Environmental factors affecting the distribution of the wild boar, sika deer, Asiatic black bear and Japanese macaque in central Japan, with implications for human-wildlife conflict. Mammal Study 34, 107–116 (2009).
-
Morelle, Chiliad., Fattebert, J., Mengal, C. & Lejeune, P. Invading or recolonizing? Patterns and drivers of wild boar population expansion into Belgian agroecosystems. Agronomics, Ecosystems & Environment 222, 267–275 (2016).
-
Oliver, W. & Leus, Thousand. Pig. The IUCN Ruddy List of Threatened Species 2008: due east.T41775A10559847. 10.2305/IUCN.UK.2008.RLTS.T41775A10559847.en. (2008).
-
Godsoe, W., Franklin, J. & Blanchet, F. G. Effects of biotic interactions on modeled species' distribution tin can be masked by environmental gradients. Ecology and Evolution vii, 654–664 (2017).
-
Ripple, W. J. et al. Status and ecological effects of the world'south largest carnivores. Science 343, 1241484 (2014).
-
Spencer, P. B. & Hampton, J. O. Illegal translocation and genetic structure of feral pigs in Western Australia. Journal of Wildlife Management 69, 377–384 (2005).
-
Skewes, O. & Jaksic, F. M. History of the introduction and present distribution of the european wild boar (Sus scrofa) in Chile. Mastozoología Neotropical 22, 113–124 (2015).
-
Gipson, P. S., Hlavachick, B. & Berger, T. Range expansion by wild hogs across the central United States. Wildlife Gild (Usa)(1998).
-
Brook, R. Thousand. & van Beest, F. One thousand. Feral wild boar distribution and perceptions of risk on the cardinal Canadian prairies. Wildlife Gild Bulletin 38, 486–494 (2014).
-
Pedrosa, F., Salerno, R., Padilha, F. V. B. & Galetti, Grand. Electric current distribution of invasive feral pigs in Brazil: economic impacts and ecological doubtfulness. Natureza & Conservação 13, 84–87 (2015).
-
Lemel, J., Truvé, J. & Söderberg, B. Variation in ranging and activity behaviour of European wild boar Sus scrofa in Sweden. Wildlife Biology 9, 29–36 (2003).
-
Lockwood, J. Fifty., Cassey, P. & Blackburn, T. The role of propagule pressure in explaining species invasions. Trends in Ecology & Evolution twenty, 223–228 (2005).
-
Sakai, A. Thousand. et al. The population biology of invasive specie. Annual Review of Ecology and Systematics 32, 305–332 (2001).
-
Warren, R. J., Bahn, Five. & Bradford, M. A. The interaction between propagule pressure level, habitat suitability and density-dependent reproduction in species invasion. Oikos 121, 874–881 (2012).
-
Syfert, M. Thou., Smith, 1000. J. & Coomes, D. A. The effects of sampling bias and model complication on the predictive functioning of MaxEnt species distribution models. PLoS Ane 8, e55158 (2013).
-
Barbet-Massin, M., Jiguet, F., Albert, C. H. & Thuiller, W. Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3, 327–338 (2012).
-
Kramer-Schadt, South. et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Diversity and Distributions 19, 1366–1379 (2013).
-
Adler, One thousand. H. & Levins, R. The island syndrome in rodent populations. Quarterly Review of Biology 69, 473–490 (1994).
-
Krebs, C. J., Keller, B. 50. & Tamarin, R. H. Microtus population biological science: demographic changes in fluctuating populations of M. ochrogaster and 1000. pennsylvanicus in southern Indiana. Ecology l, 587–607 (1969).
-
Lobo, J. One thousand., Jiménez-Valverde, A. & Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 33, 103–114 (2010).
-
IUCN. The IUCN Red List of Threatened Species. Version 2014.1. http://world wide web.iucnredlist.org. Downloaded on 26 February 2016. (2014).
-
Google Earth Engine Squad. Google Earth Engine: A planetary-scale geospatial analysis platform. https://earthengine.google.com/. (2016).
-
Hawkins, B. A. et al. Energy, water, and wide-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003).
-
Wiens, J. J. et al. Niche conservatism equally an emerging principle in environmental and conservation biology. Ecology Letters 13, 1310–1324 (2010).
-
Wiens, J. J. & Graham, C. H. Niche conservatism: integrating evolution, ecology, and conservation biological science. Annual Review of Ecology, Development, and Systematics 36, 519–539 (2005).
-
Alexander, J. M. & Edwards, P. J. Limits to the niche and range margins of alien species. Oikos 119, 1377–1386 (2010).
-
Fitzpatrick, M. C., Weltzin, J. F., Sanders, N. J. & Dunn, R. R. The biogeography of prediction mistake: why does the introduced range of the fire emmet over-predict its native range? Global Ecology and Biogeography xvi, 24–33 (2007).
-
Mau-Crimmins, T. Grand., Schussman, H. R. & Geiger, E. L. Tin the invaded range of a species be predicted sufficiently using but native-range information?: Lehmann lovegrass (Eragrostis lehmanniana) in the southwestern United states of america. Ecological Modelling 193, 736–746 (2006).
-
Loo, S. E., Nally, R. M. & Lake, P. Forecasting New Zealand mudsnail invasion range: model comparisons using native and invaded ranges. Ecological Applications 17, 181–189 (2007).
-
Broennimann, O. & Guisan, A. Predicting current and future biological invasions: both native and invaded ranges matter. Biology Messages four, 585–589 (2008).
-
Beaumont, Fifty. J. et al. Unlike climatic envelopes among invasive populations may atomic number 82 to underestimations of electric current and futurity biological invasions. Diverseness and Distributions 15, 409–420 (2009).
-
QGIS Development Squad. QGIS two.fourteen.3 Geographic Information System. Open up Source Geospatial Foundation Project. http://qgis.osgeo.org. (2016).
-
R. R: a language and surround for statistical computing, Version three.2.3. R Foundation for Statistical Computing. Vienna, Republic of austria. (Development Cadre Squad 2016).
-
Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution 1, 103–113 (2010).
-
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical data-theoretic arroyo. Second Edition., (Springer Verlag, 2002).
-
Doherty, P. F., White, Yard. C. & Burnham, Thou. P. Comparing of model edifice and selection strategies. Journal of Ornithology 152, 317–323 (2012).
-
Lukacs, P. M., Burnham, Grand. P. & Anderson, D. R. Model selection bias and Freedman's paradox. Register of the Institute of Statistical Mathematics 62, 117–125 (2010).
-
Murtaugh, P. A. Performance of several variable-selection methods applied to real ecological data. Ecology Letters 12, 1061–1068 (2009).
-
Boyce, M. S., Vernier, P. R., Nielsen, Due south. E. & Schmiegelow, F. K. Evaluating resource selection functions. Ecological Modelling 157, 281–300 (2002).
-
ESRI. ArcGIS Desktop: Version x.3.1 Environmental Systems Enquiry Institute, Redlands, CA, USA. (2015).
-
Geisser, H. & Reyer, H.-u. The influence of food and temperature on population density of wild boar Hog in the Thurgau (Switzerland). Journal of Zoology 267, 89–96 (2005).
-
Morelle, K. & Lejeune, P. Seasonal variations of wild boar Grunter distribution in agronomical landscapes: a species distribution modelling approach. European Journal of Wildlife Inquiry 61, 45–56 (2015).
-
Sweitzer, R. A. Conservation implications of feral pigs in island and mainland ecosystems, and a case written report of feral sus scrofa expansion in California. Proceedings of 18th Vertebrate Pest Briefing 18 26–34 (1998).
-
Fleming, P. J. et al. In Carnivores of Commonwealth of australia: past, present and future(eds A. South. Glen & C. R. Dickman ) (CSIRO Publishing, 2014).
-
Trabucco, A. & Zomer, R. Global soil water balance geospatial database. CGIAR Consortium for Spatial Information. Published online, bachelor from the CGIARCSI GeoPortal at http://cgiar-csi.org (2010).
-
Weltzin, J. F. et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53, 941–952 (2003).
-
Massei, G., Genov, P., Staines, B. & Gorman, M. Mortality of wild boar, Pig, in a Mediterranean area in relation to sex and age. Journal of Zoology 242, 394–400 (1997).
-
Groves, C. P. Ancestors for the pigs: taxonomy and phylogeny of the genus Sus. ane–96 (Dept. of Prehistory, Australian National University, 1981).
-
Bieber, C. & Ruf, T. Population dynamics in wild boar Pig: ecology, elasticity of growth rate and implications for the direction of pulsed resource consumers. Journal of Applied Ecology 42, 1203–1213 (2005).
Acknowledgements
This study was funded and supported by the US Department of Agriculture, Animal and Constitute Health Inspection Service, Center for Epidemiology and Creature Health, Veterinary Services, Wild fauna Services, the National Wild fauna Research Heart, the National Feral Swine Damage Direction Program, Colorado State University, and Conservation Science Partners. Nosotros capeesh the distribution data for wild pigs in Canada provided by R. Kost and R. Brook, synthesis of wild grunter distribution in Africa and South America by C. Larson, and the dingo distribution information in Australia provided by P. Fleming. P. DiSalvo and Grand. Foley assisted with acquiring literature on density estimates. M. McClure assisted with cross validation of model results. We thank three bearding reviewers, Southward. Sweeney and B. Dickson for providing thoughtful feedback that improved earlier versions of this paper.
Author information
Affiliations
Contributions
J.50. conceived the ideas, led the analyses, and wrote the manuscript. C.B., K.F., M.G., R.Chiliad., and D.T. contributed to the development of ideas, assisted with analyses, and edited the manuscript. CB created the large carnivore richness GIS layer and wild pig global range figure.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article'south Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will demand to obtain permission from the license holder to reproduce the material. To view a re-create of this license, visit http://creativecommons.org/licenses/past/4.0/
Reprints and Permissions
About this commodity
Cite this article
Lewis, J., Farnsworth, M., Burdett, C. et al. Biotic and abiotic factors predicting the global distribution and population density of an invasive big mammal. Sci Rep 7, 44152 (2017). https://doi.org/10.1038/srep44152
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/srep44152
Further reading
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you notice something abusive or that does not comply with our terms or guidelines delight flag it as inappropriate.
What Are The Biotic And Abiotic Factors That Limit Population Size?,
Source: https://www.nature.com/articles/srep44152
Posted by: staplesmarn1968.blogspot.com


0 Response to "What Are The Biotic And Abiotic Factors That Limit Population Size?"
Post a Comment